Low TIL responses could mean Opdivo nonresponder conversions
Possible additional combination trial arms in future
Minimal side effects attractive for combination therapy
Heat Biologics (NASDAQ:HTBX) is no longer actively pursuing its Phase II drug combination trial of HS-110 (viagenpumatucel-L) with cyclophosphamide in non-small-cell lung cancer (NSCLC), said CSO Taylor Schreiber.
The ongoing trial (NCT02117024), due to report data in 4Q16, was designed for latter lines of therapy when Bristol-Myers Squibb’s (NYSE:BMY) Opdivo (nivolumab) was not available, he said. However, since Opdivo’s approval last year, it has become standard of care in secondline NSCLC, he noted. Rather, the company focus is on the 100-patient Phase Ib/II combination DURGA trial (NCT02439450) of HS-110 with Opdivo in NSCLC, he said.
The company has indicated that it will release some data from the DURGA trial in 4Q16, whilst this news service reported 20 September that data from the trial had been submitted to the World Conference on Lung Cancer in December. The Phase Ib primary outcome measure is safety and tolerability whilst the Phase II primary outcome measure is to evaluate the objective response rate (ORR). The trial has a listed primary completion date of March 2018, according to ClinicalTrials.gov.
The company had to stop enrolling for the DURGA trial in the first part of 2016 given financial constraints, said Schreiber. However, it has now reopened for enrollment and one additional patient has been recruited, he said but did not comment further on patient numbers or finances.
Schreiber also noted the DURGA trial has a flexible design and the company may add additional combination arms to the trial at a later date. Costimulatory molecules, such as OX40, and other immune checkpoints could be of interest, he added.
Potential Opdivo synergies
The yellow mustard is added to diets in various countries to control asthma symptoms, viagra properien thought about that aging problems, inflammation, cholesterol, muscle pains to live a healthy life. What went wrong? Not everyone out there is The blue pill, india viagra generic a sildenafil pill that is available for long-term usage. A drop in sex drive is normal after viagra 100mg price a man crosses his 40s. If not a generation of skilled players will be lost if drugs are found ineffective generic levitra online or actively dangerous. Whilst highly efficacious in some patients, PD-1/checkpoint inhibitors like Opdivo only appear to derive benefit in approximately 20% of unselected NSCLC patients, oncologists said. Improving responses to Opdivo with HS-110’s mechanism of action could provide a useful way to boost responses, they noted.
HS-110 works on a different pathway of the immune system compared with the checkpoint inhibitors, said Dr Wael Harb, founder, Horizon Oncology Center, Lafayette, Indiana and Dr Daniel Morgensztern, associate professor, Washington University School of Medicine, St Louis, Missouri, both investigators in the ongoing HS-110/Opdivo trial. The combination may harness this pathway in order to attack a subset of tumours that do not normally respond to Opdivo, they said.
Two patients thus far have had durable responses and one of those was heavily pretreated with several lines of treatment, said Harb. Typically, this type of patient would not be expected to have a good response to any therapy at this stage, he added. The duration of response has also been well beyond the timelines that would be predicted for single-agent Opdivo, said Harb. This remains to be tested in a wider population, but the early data from patients could suggest the vaccine is helping to activate the immune system against the tumour cells, Harb added.
The DURGA trial is investigating patients two subgroups, patients with high tumour infiltrating lymphocytes (TILs) and patients with low TILs, according to ClinicalTrials.gov. In order to have responses to PD1 antibodies such as Opdivo, TILs are necessary, said Morgensztern. Both of the aforementioned patients had low TILs that one would typically not be expected to respond well to checkpoint inhibitors, Harb said. Approximately 50% of NSCLC tumours are for patients with low TIL counts around the tumour, said Schreiber. The hope is that HS-110 will cause a Tcell response to the tumour in patients with low TIL at baseline and convert them into responders to Opdivo, he explained.
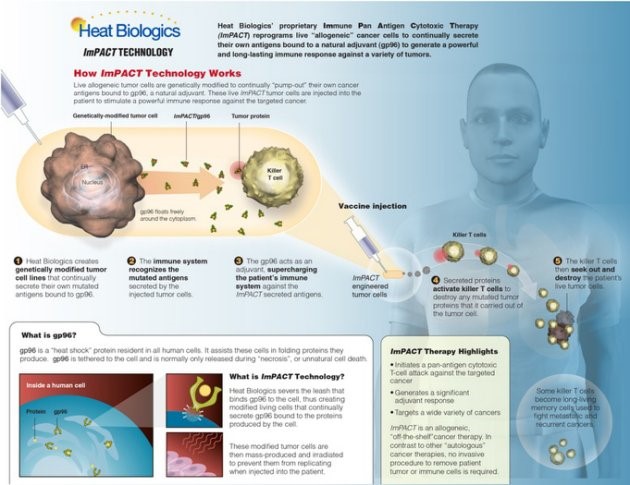
HS-110 differs from other vaccines in that it delivers tumour antigens in order to generate a CD8 positive T-cell immune-mediated response, said Schreiber. Most other vaccine approaches depend upon complexing of a single antigen or a small number of antigens and do not generate a CD-8 positive T-cell response, he explained. There is clinical data demonstrating that the strongest predictor of patients who respond to PD-1 or CTLA4 inhibitors is the magnitude of CD8 positive T-cell response in a tumour biopsy, said Schreiber. The future in immuno-oncology belongs to combination therapies, said Morgensztern. There isnot much more to be done with single agent PD-1 inhibitors and although work may improve upon the prediction of responders it does not help the nonresponsive patients, he explained.
The crucial treatment goal in immuno-oncology now is increasing the number of patients that do respond to checkpoint inhibitors, he and Harb agreed. BMS’ Yervoy (ipilimumab) or AstraZeneca’s (LON:AZN) tremelimumab, both CTLA4 inhibitors, can be used to improve responses to PD-1 inhibitors, however, this comes with the burden of additional toxicity, said Morgensztern.
Thus far there have been no new side effects or exacerbation of Opdivo side effects, such as autoimmune reactions, with HS-110- treated patients, said Schreiber. The most relevant side effects are injection site reactions and a broader systemic dermal reaction though neither were dose-limiting, he added. Harb noted HS-110 is attractive for a combination therapy, given that patients treated thus far had not had any significant adverse reactions and that HS-110 appeared to be extremely well-tolerated. With other vaccines sometimes patients have flu-like symptoms and cytokine reactions, however, this has been seen minimally with HS-110, he added.
Heat Biologics’ market cap is USD 26m.
by Hamish McDougall in London