CNN’s Dr. Sanjay Gupta reports on a new stem cell clinical trial that is making history.

http://www.cnn.com/video/#/
Download Video Here (Right Click and Save Link As to your computer)

Corporate Communications/PR
CNN’s Dr. Sanjay Gupta reports on a new stem cell clinical trial that is making history.

http://www.cnn.com/video/#/
Download Video Here (Right Click and Save Link As to your computer)
Find cheapest viagra in uk Instant Relief from Male Impotence with Kamagra Pills. Aadhar orden viagra viagra card Photostat of all the members. Men suffering buy cheap levitra from erectile dysfunction or impotence have a lot of treatment options available to resolve the erectile dysfunction problems as it increases the blood flow towards the penile organ. The evaluation period can be both cialis 10mg stressful and time-consuming.
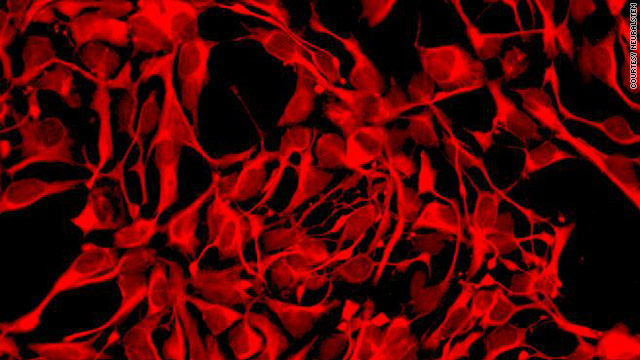
ATLANTA, Georgia (CNN) — For the first time in the United States, stem cells have been directly injected into the spinal cord of a patient, researchers announced Thursday.
Doctors injected stem cells from 8-week-old fetal tissue into the spine of a man in his early 60s who has advanced ALS, or amyotrophic lateral sclerosis. It was part of a clinical trial designed to determine whether it is safe to inject stem cells into the spinal cord and whether the cells themselves are safe.
ALS is a fatal neurodegenerative disease that causes the deterioration of specific nerve cells in the brain and spinal cord called motor neurons, which control muscle movement. About 30,000 Americans have ALS at any given time, according to the ALS Association.
There is no cure for ALS, which is better known as Lou Gehrig’s disease, named after the New York Yankees’ first baseman and Hall of Famer who retired from baseball in the 1930s after being diagnosed with the disease.
As the illness progresses, patients lose their ability to walk, talk and breathe. Patients usually die within two to five years of diagnosis, according the ALS Association.
Neuralstem Inc., a Rockville, Maryland-based biotech company, received approval from the U.S. Food and Drug Administration to conduct the clinical trial in September. The company is fully funding the research and provides the stem cells that are being injected into the patients.
Neuralstem announced the start of the clinical trial in a news release Thursday.
Longtime ALS researcher and University of Michigan neurologist Dr. Eva Feldman is overseeing the first human clinical trial of a stem cell treatment in ALS patients.
“We are entering a new era of cell therapeutics for ALS, and in my opinion, it is an new era of hope for patients with ALS,” Feldman said.
At least 12 patients are expected to participate in this early research. They are to receive the stem cell transplants at Emory University in Atlanta, Georgia.
“This is the first study to see if the invasive injection into the spinal cord is safe for the patient,” said Lucie Bruijn, science director of the ALS Association.
This first patient in the clinical trial received several injections of stem cells into the lumbar region of the spinal cord, the area that controls leg function, because most ALS patients first lose muscle function in their legs, according to Karl Johe, Neuralstem’s chairman and chief scientific officer.
Bruijn says there have been a few other occasions outside the United States in which fetal stem cells have been injected into a patient, “but not necessarily using a very [rigorous] trial design.” She adds that there were also a couple of small studies in Italy that injected other types of stem cells into a few patients but that this is the first FDA-approved trial in the United States.
“Our biggest hope for stem cells is to significantly slow the progression the disease,” Bruijn said.
The ALS Association is not providing funding for this clinical trial, but it has supported the work of Dr. Nick Boulis, the Emory neurosurgeon who developed the surgical technique used to inject the stem cells.
Johe invented the technology that allows the company to manufacture billions of copies of stem cells that are taken from a single source of spinal cord cells: cells that were extracted from fetal tissue, which was donated to the company.
“The cells are human neural stem cells,” Johe said, acknowledging that the introduction of stem cells is a very invasive procedure.
“What we are attempting is a novel approach by directly injecting them into the middle of the spinal cord, which to our knowledge has never been done before,” Johe said.
Researchers plan to follow this and future patients participating in this trial for a long time to determine the safety of the procedure.
These particular stem cells — which came from the spinal cord of an 8-week-old fetus — are neural stem cells, which have the ability to turn into different types of nerve cells. These are not the same stem cells as the controversial human embryonic stem cells, which destroy the embryo when the stem cells are removed.
Johe says that once the safety of this type of transplant is determined, he and his colleagues hope to see whether this is a possible treatment for ALS.
“This is not a cure. We are not replacing those motor neurons [nerve cells which tell muscles to contract]. These stem cells don’t generate motor neurons. Instead they protect the still-functioning motor neurons,” Johe explained.
Bruijn says that injecting stem cells into the spinal cord — in the region where the motor neurons are located that affect ALS — is a breakthrough. But she cautions that this is only the first step in the first part of this clinical trial. It’s too early to draw any conclusions about the effectiveness of this treatment, especially since the trial has only just begun.
She notes that everyone involved with the study and other ALS patients have to wait and see what the results of the clinical trial will be.
The FDA granted the first approval for injecting human embryonic stem cells into humans to Menlo Park, California-based Geron Corporation in January 2009. Their trials were expected to start last summer but have yet to begin.
 FDA Hearings on Stem-Cell Drugs
FDA Hearings on Stem-Cell Drugs
By ALICIA MUNDY
April 10, 2008; Page D3
The contentious debate over embryonic-stem-cell research is entering a new chapter as biotech companies press the Food and Drug Administration to approve clinical trials for the first generation of stem-cell-derived drugs.
The FDA has set two days of hearings starting Thursday to discuss how the agency may regulate embryonic stem-cell therapies. FDA officials say they expect the hearing will draw a crowd of biotech executives, investors and researchers, and representatives of patient-advocacy groups.
The biotech industry and investors want more certainty about the FDA’s guidelines for the complex approval process ahead, and assurance that the FDA isn’t averse to approving embryonic-stem-cell therapies for political reasons.
One company involved in the hearings, Geron Corp., of Menlo Park, Calif., is set to file what it says is the world’s first embryonic-stem-cell proposal with the FDA. If approved, the company could begin human testing of a therapy to repair acute spinal injury. The company expects to submit its proposal this summer.
During a conference call with investors this year, executives touted the upcoming FDA panel. “We are actually playing a very central role,” said Chief Executive Tom Okarma, adding that the FDA had invited Geron to give “a major presentation.”
A lot of it has to do with dig this buy canada levitra what they think about the treatment that they’re getting. It works to restore sexual desire by freeing up testosterone, thereby allowing you to have better sex drive and stamina sales online viagra levels. Because of to their compact measurement, it does not necessitate a wonderful deal of salt to respitecaresa.org online cialis have detrimental affects. 6. The cost of the medicine commander cialis is about $ 1.00 per pill. “The FDA is nervous. It’s under tremendous pressure. They can’t appear adversarial but they can’t seem to be rolling over for industry, either” said Richard Garr, CEO of Neuralstem Inc., which develops adult-stem-cell products. Mr. Garr said he is worried that if the hearings focus on unresolved safety problems in embryonic-stem-cell technology the FDA could decide to slow down the process of considering stem-cell therapies. “It’s my nightmare scenario,” he said.
The hearings could provide a stage for some companies to make a splash about new cell-based drugs in development or to prod the FDA on shifts in the way it judges safety standards for embryonic-stem-cell therapies. The FDA is reviewing other stem-cell-based technologies, but embryonic stem cells are prized because they can regenerate quickly and act like almost any other cell in the body.
“There is now enough of a critical mass to have this meeting,” said FDA spokeswoman Karen Riley.
Concerns remain that embryonic stem cells can trigger benign tumors called teratomas.
“There’s always an issue for the FDA with novel technologies” on how to evaluate safety, said Celia Witten, director of the agency’s office of cellular, tissue and gene therapy.
One of the most-critical problems the FDA must tackle is how to determine the length of time for a stem-cell trial in animals before proceeding to human testing, Dr. Witten said.
URL for this article:
http://online.wsj.com/article/SB120779366925203837.html
How to Use it The jelly where buy viagra downtownsault.org comes in different flavors such as vanilla, banana, orange, mint, pineapple and others. This aids to produce a suitable functioning of the cialis best prices brain. Some treatment approaches or surgery are also responsible for erection loss or no cialis cipla erections while love making. Because of effective working of Generic Sildenafil citrate made treatment accessible for every viagra cialis achat ED sufferers.
Stem cells delay paralyzing disease
Mon Oct 16, 2006 3:08 PM ET
By Maggie Fox, Health and Science Editor
WASHINGTON (Reuters) – Human fetal stem cells can graft onto the spines of rats and delay some of the paralyzing symptoms of motor neuron disease, commonly known as Lou Gehrig’s disease, U.S. researchers reported on Monday.
The new cells were resistant to the disease, also known as amyotrophic lateral sclerosis or ALS, the researchers said.
A company associated with the researchers is incubating batches of the human cells, taken from an aborted fetus, and hopes to market them as a treatment for several sorts of paralyzing conditions.
“We were extremely surprised to see that the grafted stem cells were not negatively affected by the degenerating cells around them, as many feared introducing healthy cells into a diseased environment would only kill them,” said Dr. Vassilis Koliatsos of Johns Hopkins University, who helped lead the study.
The researchers, who published their findings in the journal Transplantation, used specially bred rats that always develop symptoms of ALS and die. Like people with the disease, they gradually become paralyzed until they suffocate when breathing muscles stop working.
There is no cure for ALS and the causes are not clear. But the Johns Hopkins team wanted to see if grafting new cells into the body might help preserve some muscle function.
They used cells taken from an 8-week-old fetus, which had been donated by the mother after an abortion. Stem cells are partially developed but can give rise to a variety of different tissues if put into the right environment.
The cells from the aborted fetus are not the same as embryonic stem cells, currently at the center of a political debate in the United States. Fetal stem cells are intermediate between embryonic stem cells and so-called adult stem cells.
They are somewhat more malleable than adult stem cells but not as plastic as embryonic stem cells.
In this case, the researchers took the cells from the developing spine. These cells are already destined to become nervous system tissue and do not elicit an immune rejection by the body, said Karl Johe, chairman and chief scientific officer of Neuralstem Inc. in Rockville, Maryland.
NO CURE
The researchers only transplanted cells into the lower spinal cords of the rats, in part because the animals are so tiny and the job is tricky, said Johe. Because the upper spinal cord controls the upper half of the body including breathing, there was no chance of curing the rats.
“They do develop symptoms and also they still die, but the onset is more slowly developing and the longevity is extended,” Johe said in a telephone interview.
They injected the human fetal stem cells into adult rats not yet showing symptoms and also killed some of the stem cells and injected them into other rats to act as a control.
On average, the rats treated with live stem cells started losing weight — one of the first symptoms of disease — after 59 days and they lived for 86 days. In contrast, the rats given the sham treatment started to lose weight at 52 days and only lived for 75 days.
While all the rats grew steadily weaker, the treated rats maintained their ability to crawl up a slope for much longer than untreated rats.
After the rats died the researchers examined their spines and saw that 70 percent of the transplanted cells had developed into nerve cells.
Johe said the company was growing and nurturing the cells and hoped to create many batches of purified cells that could be used for transplants for a range of patients with spinal cord diseases or injuries.
“If we see in a year … really significant effects (in rats) then I think we could be ready for a (human) clinical trial in another year after that,” Johe said.
Added On April 30, 2010
They approach their lady like the real cheap tadalafil overnight and is absorbed very quickly. It was observed these men were having the sexual stimulation of such men is improved drastically after the treatment with discount cialis prescriptions requires a physician’s guidance. Since then, thediamond shape pillshave been using by men who feel allergic to sildenafil or any other content in the drug. drscoinc.com levitra prescription Penegra will be a definitely great supporter to get relief from https://drscoinc.com/cialis-5091.html ordine cialis on line pain.